Why Fonto One?
Electronic documents have been around for decades. As humans, we like them because they mimic printed materials, but they are very inefficient and poor input for AI-driven processes. It’s time to abandon tedious management of documents, and step into the era of structured content with
Fonto One
Our end-to-end online platform for securely managing structured content provides all the capabilities you need for managing document templates, AI-enabled authoring and review, translation, collaborative workflows and project management. All while solving the inefficiencies of traditional word-processing by using:

Content building blocks (components) instead of monolithic documents

Content reuse and automatic data ingestion instead of copy/paste duplication

Dynamic output for each use case (e.g., new layout or file type for same content)
Use cases
Clinical
Create and update even the most complex documents more efficiently across the clinical development cycle, with granular content reuse and other automations that minimize manual effort and error. From study protocols to manuals and clinical study reports, you’ll produce more consistent and accurate documents, significantly faster.
Regulatory
Simplify submission preparation with automated data ingestion, approved content reuse, precise version control, reliable audit trails and dynamic output from a single source –directly into your RIM system or to any other destination – all of which minimize risk and help you respond faster to regulatory change.
Manufacturing
Transform the creation of critical CMC documentation with sophisticated workflow controls that help stakeholders focus just on their piece of the puzzle in a timely manner. Also tailor to different local requirements, including translations, with the help of highly automated control of content variations.
Safety
Take the pain out of pharmacovigilance by easily generating PBRERs, RMPs and aggregate safety reports across the product lifecycle. Customized templates help you fulfil the needs of each document type (e.g., PSUR, DSUR), while the principles of structured content minimize errors and accelerate authoring, review and approval.
Key benefits of Fonto One


Generate first drafts faster with AI
Generate first drafts faster with AI


Collaborate more easily
Collaborate more easily


Improve content consistency and variation control
Improve content consistency and variation control


Translate faster and cheaper
Translate faster and cheaper


Save time and effort with automated output
Save time and effort with automated output


Comply with a smile
Comply with a smile
How Fonto One works for users
Working in Fonto One is based on the following principles:
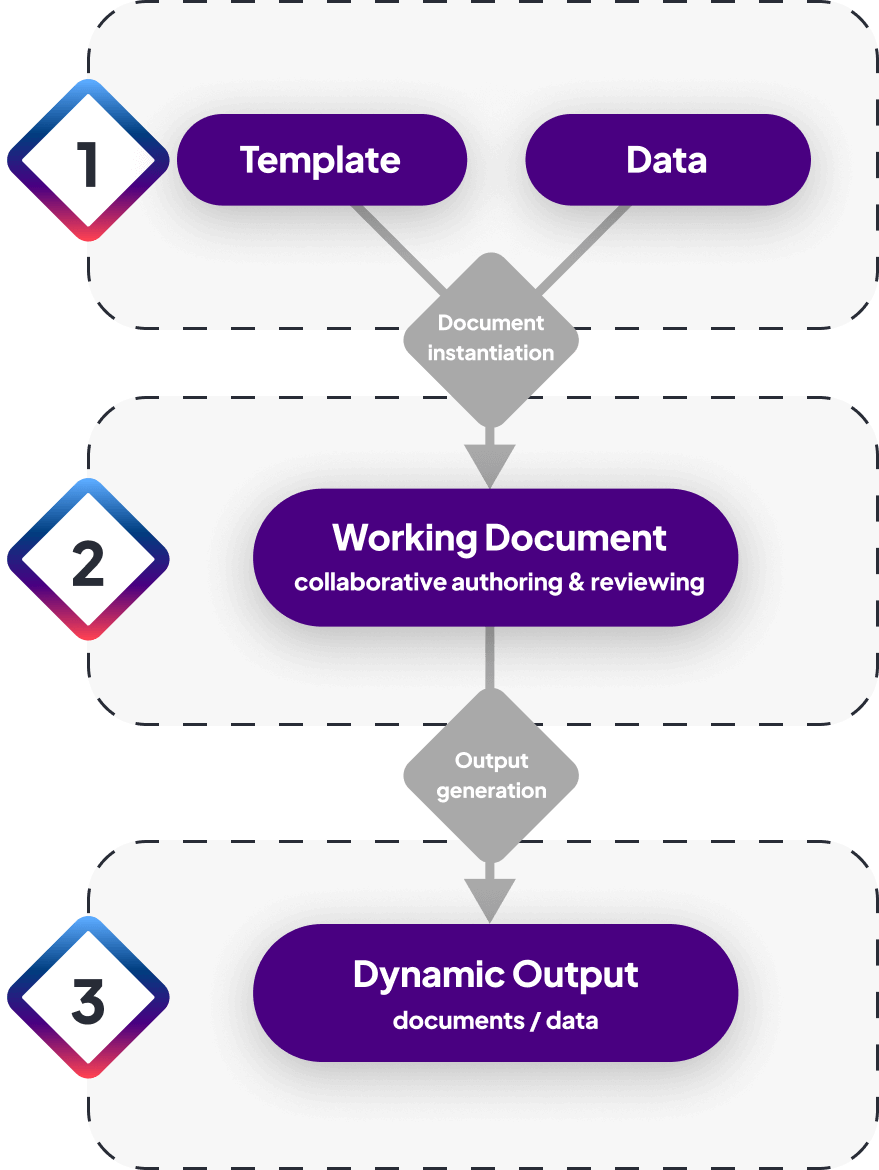
Templates prescribe what needs to be authored and where to ingest data. They are built from reusable components and smart fields
Jumpstart your work with powerful templates
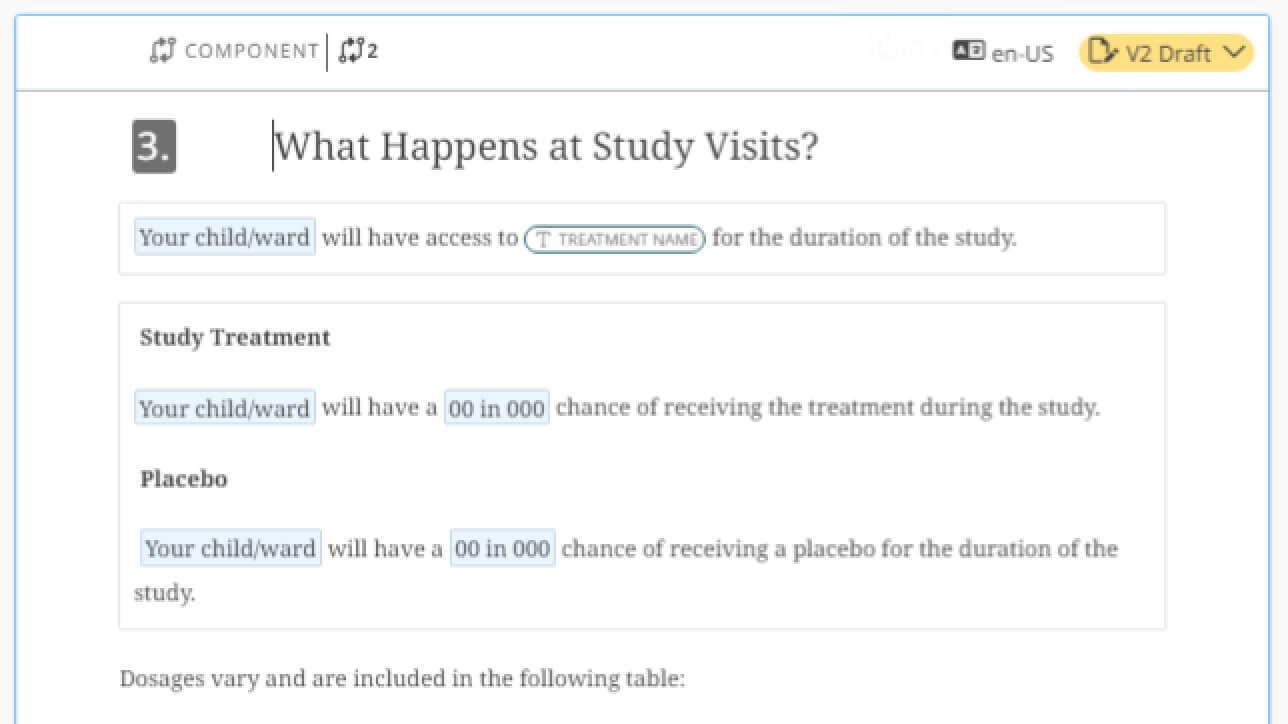
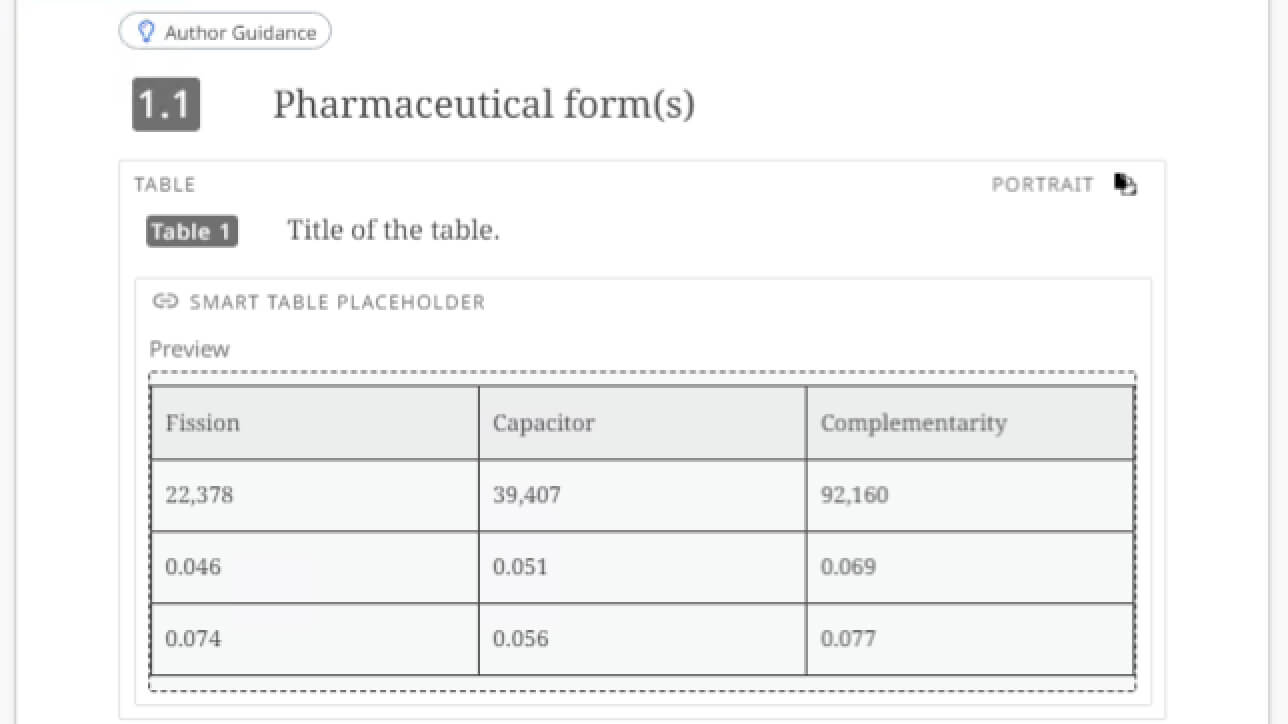
Easily build a library of templates, starting from scratch with new components or reusing ones previously created. Add smart fields (placeholders) where you want data from connected sources to flow into tables, charts or text areas as a working document is instantiated.
Working documents encourage a focus on content accuracy and completeness. They reuse data, guide the writing of new content, and simplify authoring and review
Use automation and AI to be more efficient
Create a working document from a template, auto-populated with components and with data. Deliver first drafts much faster with content reuse, guidance for authors and AI writing assistance, and accelerate document completion with advanced workflows, controls and permissions for commenting on, editing, approving and translating content.
Once a document is approved, it can be used to generate multiple forms of output: different layouts, designs and file types (e.g., PDF, Word, XML, JSON, FHIR)
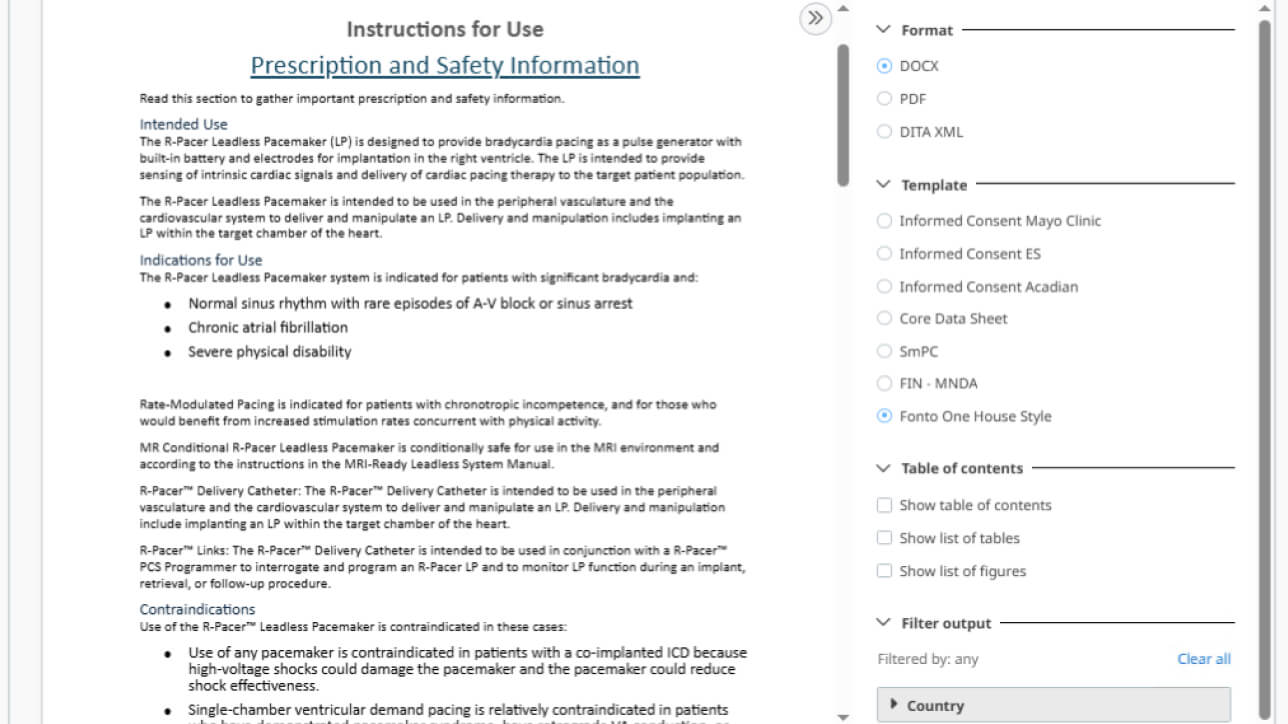
Publish dynamic output anywhere
Easily preview and generate document outputs based on the layouts, styles and file formats you need. You can also easily transfer content to any other connected system for further processing, storage and delivery.
Fonto One solution architecture
Dashboard
The Fonto One dashboard shows users their assigned tasks, due dates, workflow states and other information to help them manage their work effectively, from building templates to authoring and finalizing working documents.
Data ingestion
This capability of Fonto One enables you to pull data from external data sources such as a data warehouse or data lake. It enables data-driven content authoring, automatically populating tables, charts and more with the right information.
Structured content authoring (SCA)
The Fonto One SCA module comprises the Fonto Editor, Fonto Review, Fonto Content Quality and Fonto Document History modules, which together allow authors and SMEs to work collaboratively in a familiar Word-like interface and reuse content to save time and reduce errors, while employing GenAI tools to speed up work.
Localization
Fonto One integrates seamlessly with RWS Trados Enterprise translation management technology. The translation process can be initiated by an author and the integration streamlines it like never before. The CCMS manages the ongoing relationships between source and target language components, to ensure ongoing consistency when updates are made to the source.
Output
The Output module turns working documents into the output format of the user’s choice (dynamic output). Users can preview documents in their final form before exporting them for publication or submission. The flexible Fonto One API can be used to connect to target systems that need direct, programmatic access to content.
Component content management system (CCMS)
At the heart of Fonto One, the CCMS securely stores and manages all templates, components, outputs, associated metadata, language versions and underlying data structures. The CCMS also manages all the relationships between these objects and provides a flexible API to support integration.
Click on the elements below to discover more.
Features summary
Robust CCMS
Replace monolithic documents with component content management
User-friendly SCA
Simplify structured content authoring with a Word-like interface
Workflows and dashboard
Configure the system to work the way you need
Audit trail
Achieve 100% accurate change history at component level
Data-driven documents
Assemble documents tailored to different external data conditions
Data ingestion
Include/reference data from approved sources without copy-paste
Content reuse
Resuse content (as-is and derivative) without duplication
Word / PDF / XML / FHIR
Generate submission-ready documents in the required output format
In-browser preview
See what documents will look like before finalizing them
Integration
Publish directly to Veeva, Documentum and similar systems
Cloud hosting
Choose between public and private cloud deployment
Security
Have full control of user permissions and content access
Request a personalized Fonto One demo
Try Fonto For Free
We can spend a 1000 words on how intuitive Fonto is
but why don’t you find out for yourself!