In the ever-evolving landscape of pharmaceutical regulations and information management, the need to standardize and harmonize product information has become increasingly crucial. At the forefront of this movement is the Swedish Medical Products Agency, Läkemedelsverket, which has recently made significant strides with their ePI (electronic Product Information) Proof of Concept. In this post, we’re sharing the latest developments in this transformative initiative.
Creation of electronically structured product information
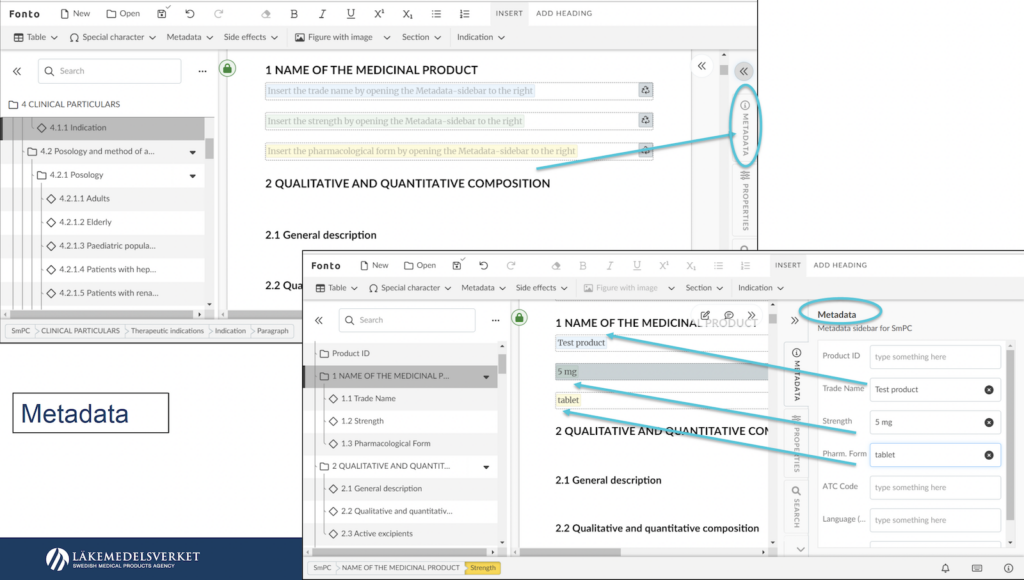
Earlier this year, we reported on the actual developments of the ePI Proof of Concept by the Swedish Medical Products Agency, Läkemedelsverket. In a series of workshops conducted, Läkemedelsverket presented the ePI concept to representatives from ten pharmaceutical companies. These industry experts had the opportunity to create electronically structured product information using the Proof-of-Concept configuration of Fonto Editor, customized to their specific XML scheme and template.
The workshop participants concluded that the ePI concept not only enhances the willingness to structure information but also promotes standardization and harmonization, leading to improved utilization of other e-health data. Kim Sherwood, the project manager at the Swedish MPA, highlighted the potential benefits of this concept in streamlining processes and facilitating the combined use of product information with other crucial healthcare data.
Comparable project at the European Medicines Agency
While Läkemedelsverket’s ePI Proof-of-Concept sets the stage for transforming product information management, a parallel project is underway at the European Medicines Agency (EMA). The EMA project focuses on developing a tool and portal for semi-structured product information, encompassing headings, sections, and even FHIR (Fast Healthcare Interoperability Resources) functionality. Although the EMA project lacks the code relationship and metadata functions found in the Swedish MPA initiative, both projects share a common goal of advancing product information management practices.
As the Swedish MPA progresses with their national project and collaborates with the EMA initiative, the aim is to merge the two efforts, leveraging the insights and experiences gained from the national project to enhance the European-wide solution. However, this transition will be a gradual process, given the challenge of converting approximately 50,000 documents into the new format.
To ensure a comprehensive approach, the Swedish MPA has engaged with various stakeholders, including the pharmaceutical industry, pharmacy representatives, patient organizations, and the Swedish healthcare system. By fostering collaboration and incorporating diverse perspectives, the goal is to shape a standardized and harmonized framework that benefits the entire healthcare ecosystem.

Customer Success Manager at Fonto – Passionate runner and Dad
